Abstract
Background
Patients with Sickle cell disease (SCD) suffer from frequent vaso-occlusive crises (VOC) that heavily contribute to morbidity and mortality. VOC's constitute the principal cause of emergency department (ED) presentation and hospital admission and the ED visit serves to diagnose underlying disorders, VOC complications and to institute pain relieve most often requiring intravenous opioids. The need for prompt and adequate treatment and pain relieve is reflected by standardized protocols for acute care in SCD. Adherence to these protocols has been shown to increase the quality of care, while improving overall patient pain control, decreasing frequency of ED visits and ED patient crowding and decreasing total hospital days. Several studies have demonstrated that negative health care provider attitudes interfere significantly with adequate assessment of pain and in turn may lead to insufficient treatment. We recently (October 2019) introduced novel protocols for management of SCD patients presenting with VOC's at the ED, but local the actual protocol adherence has not been evaluated.
Aim
Retrospective analysis of adherence to ED protocols for management of SCD patients on the ED.
Methods
We included all patients with SCD presenting at the ED between March 2021 and March 2022. Children and patients that presented for alternative causes than a VOC were excluded from analysis. The SCD protocol contained a circumscriptive manual with indicated timing of interventions that could be analysed for adherence. Interventions were divided into three groups: diagnostics, supportive care and analgesics. Diagnostics contained physician consultation within 60 minutes of arrival, measurement of vital parameters (SpO2, respiratory frequency, heart rate, blood pressure and body temperature), and extensive laboratory testing. In patients with chest pain it prescribed chest imaging and arterial blood gas analysis. Supportive measures included oxygen supplementation in hypoxic patients and administration of intravenous hyper hydration in absence of contraindications such as a history of fluid overload. Analgesia included treatment using a predefined personalized pain plan (PPP) if available. In all other patients, treatment consisted of acetaminophen, NSAIDs and opioids until the NRS pain score was 6 or lower or pain was deemed acceptable by the patient. Opioid treatment consisted of IV fentanyl in a maximal cumulative dose of 2 mcg/kg within the first hour followed by opioid escalation with intravenous (IV) morphine in a maximal cumulative dose of 50mg. In patients with renal failure (eGFR<30 ml/min), buprenorphine was advised instead of morphine. When analgesic treatment remained insufficient after 15 minutes the protocol prescribed consultation of the pain specialist for administration of clonidine or esketamine.
Results
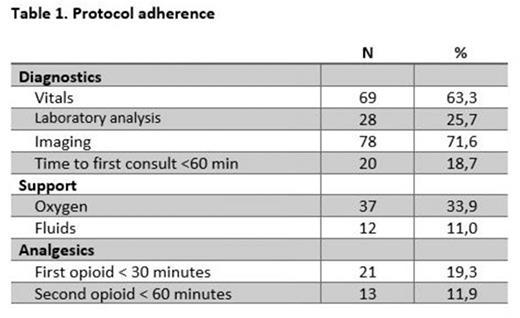
We analyzed data from a total of 109 ED visits of 70 unique patients (median age: 31,1 years (IQR 14,0), 49.5% male) between March 2021 and March 2022 in a tertiary teaching hospital in The Netherlands. Results of protocol adherence are shown in table 1. Adherence to the complete protocol was extremely low (0.9%). For most individual components of the protocol adherence was above 50%. For fluid administration, timing of initial fentanyl and morphine escalation, adherence was 39.4%, 19.3% and 11.9%, respectively.
Conclusion
In patients with sickle cell disease, protocols aim to increase accuracy and speed of diagnostics and analgesia which is related to outcomes such as overall patient pain control, frequency of ED visits and total hospital days. This study evaluates protocol adherence in a large tertiary university hospital. Whereas adherence to individual components of the SCD is above 50%, total patients in which complete protocol adherence is observed is extremely low. We identify fluid administration and opioid administration as protocol components to which adherence is most insufficient. Further research on the reasons for these impaired adherence together with training of medical and nursing staff is required to improve the quality of healthcare for this patient group.
Disclosures
Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Biemond:CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Sanquin: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal